Personalized Cancer Care with FoundationOne CDx
Table of contents
Table of contents

The term “cure for cancer” is misleading as it implies there is one single problem that needs to be solved in order to eradicate all cancers. This couldn’t be further from the truth. There are 5 main cancer groups which are further subdivided into more than 200 different types of cancer. There won’t be a single cure but rather a combination of “cures” that eventually make cancer benign. One of these “cures” is early detection, something that Illumina (NASDAQ:ILMN) is eyeballing with their startup called “Grail” which aims to develop a blood test to detect cancer “early before symptoms appear offering higher survival rates compared to late-stage diagnosis“. Maybe we’ll eventually get to a place where your smart toilet detects cancer in your urine and tells your smart fridge to sort you out by putting some drugs in your morning smoothie. Until that time though, many people who are already afflicted with cancer are asking how they might increase life expectancy using the latest technologies on offer. The answer to that question just might be “personalized medicine”.
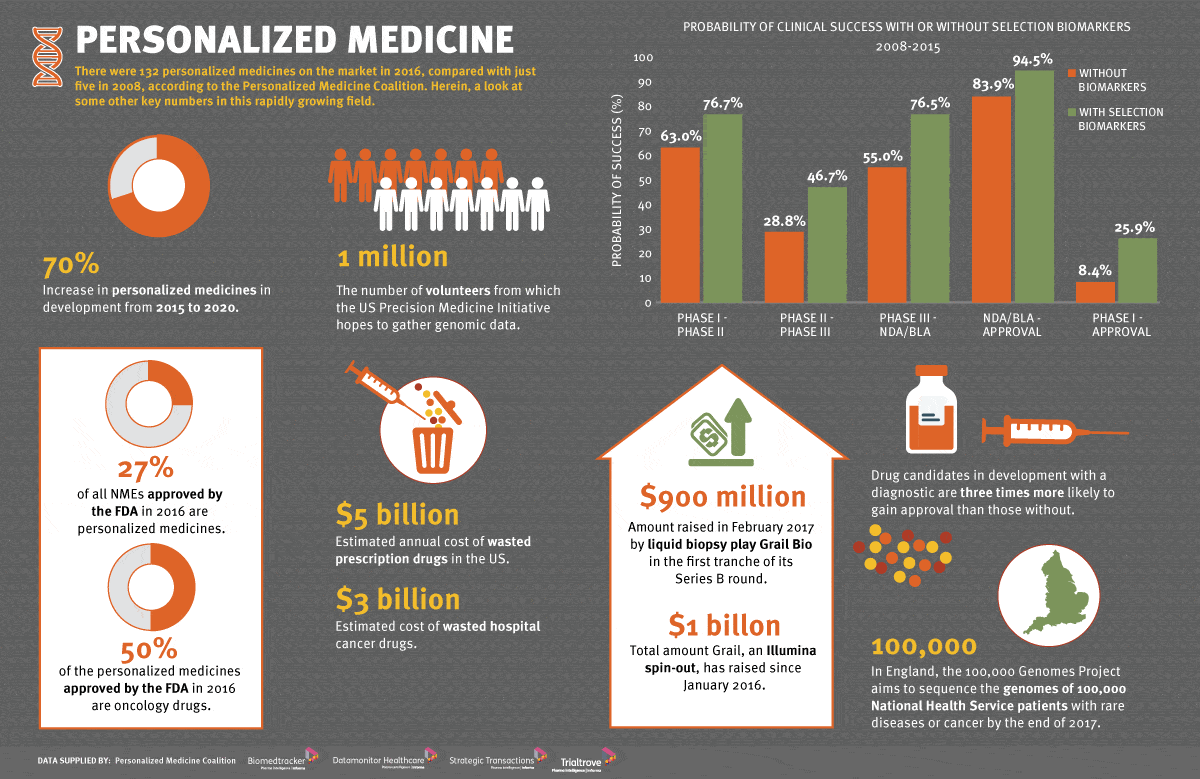
A constant challenge doctors face is trying to decide what treatments will be most effective for any particular cancer patient. Each cancer tumor is unique in that it contains certain genetic biomarkers. In simpler terms, we can use the same sort of sequencing techniques used for figuring out who your ancestors were to look at the genomic makeup of a cancer tumor and see if it has similar attributes to other tumors we’ve seen. The process is called “cancer genome sequencing” or “Next Generation Sequencing (NGS) tumor profiling” and involves taking a tissue biopsy of the tumor and analyzing it with some of Illumina’s sequencing machines. We can then look for the existence of certain biomarkers called “cancer genes” which make a tumor unique.
Once we are able to probe the existence of cancer genes in each unique tumor, we can then start to experiment with drug combinations for various tumor types to see which treatments are most effective for any combination of genes. One company we’ve talked about before which is working on tumor profiling is Foundation Medicine (NASDAQ:FMI). Just a few weeks ago, FMI made the news with a landmark FDA approval. Their FoundationOne CDx genomic profiling test for solid tumors became the first and only FDA-approved test of its kind for solid tumors (more on this claim later). Here’s a look at a sample report from the test:
That’s pretty straightforward to understand. The “genomic findings” section maps to an “FDA-approved therapeutic options” section. Presently the test can suggest 17 different FDA-approved targeted therapies, 12 of which are “first-line therapies” which means they should always be tried first based on historical success rates. Today, 50% of all new cancer drugs being developed are projected to be related to a biomarker. We would expect to see the list of biomarkers grow alongside the list of “targeted therapies”, a term we should quickly explain a bit more.
Traditional chemotherapy treatments were crude in that they threw the baby out with the bath water. It’s the same reason why these treatments had such bad side effects. They killed healthy cells right alongside cancerous cells. Now, cancer therapies are being developed to attack specific molecular targets, something we also refer to as “precision medicine”. In the future, the first thing you’ll do is get a biopsy in order to determine the unique genetic composition of your tumor. Then, you’ll be provided with a recommended treatment based on all available information to date. In addition to FDA-approved targeted therapies, you will also have the option to participate in clinical trials. All of this will be made possible by “big data”, or in this case, lots and lots of tumor data.
Over time, Foundation has put together a molecular information knowledgebase that contains genomic information from more than 120,000 patients whose tumors have been profiled by their tests. That database will prove to be one of their most valuable assets going forward since they are not the only ones with such a test. Memorial Sloan Kettering Cancer Center (MSK) has developed their IMPACT test which also “uses next-generation sequencing (NGS) to rapidly identify the presence of mutations in 468 unique genes, as well as other molecular changes in the genomic makeup of a person’s tumor“. About a month ago, the FDA granted approval for the test along with “a policy framework that paves the way for the efficient review and availability of other NGS-based cancer profiling tools“. You can read more about the whole thing in this FDA announcement (15 days before the Foundation approval we might add), but the key takeaway here is that more tests will be coming to market. Even Illumina has their own tumor profiling product which shouldn’t come as much of a surprise considering their lofty ambitions with Grail.
While FMI’s tests are quite broad, there are lots of granular tests that can be used to detect certain mutations known as “hotspot testing”. There is a great article on ASCO Connection which talks about the difference between “hotspot testing” and “NGS tumor profiling”. Here’s an excerpt (note that NSCLC refers to a type of lung cancer):
Hotspot testing in advanced NSCLC focuses on testing for unique, identified gene alterations that have been correlated with an effective targeted therapy, while more broad-based testing with next-generation sequencing (NGS) looks at many more potential gene alterations, up to several hundred genes, to characterize the tumor genetic profile. At this time, however, the utility of NGS sequencing as initial testing for all patients with NSCLC has not been proven.
We might have a bone to pick with that last sentence. In the case of the FoundationOne CDx test, 12 of the targeted therapies are actually “first line” therapies which means an initial test would make sense. You could argue that since 2 out of 3 patients won’t find the test actionable, it doesn’t make sense to take. Then try arguing that while pretending that you’re the one with cancer and see if you still feel the same way. Here’s what FMI says about comprehensive genomic profiling versus single-gene “hotspot” testing for solid tumors:
Single-gene testing identifies only one or two classes of genomic alterations. Comprehensive genomic profiling (CGP) approach identifies four classes of genomic alterations: base substitutions, insertions and deletions/indels, copy number alterations, and rearrangements.
It’s also worth mentioning that the Foundation test does some other useful things like “microsatellite instability (MSI) and tumor mutational burden (TMB) that can help inform the use of immunotherapies”. Immunotherapy was a topic we touched on a few years ago in an article titled “Investing in Cancer Immunotherapy Companies“. While some of the prior concerns about Foundation Medicine’s business model were the cost of the test, the recent FDA approval came with news of a national coverage determination (NCD) being under review. According to an article by GenomeWeb on the topic:
“Once final, the NCD will represent a significant expansion of coverage for Medicare beneficiaries nationwide by initially covering five tumor types,” Cox said. Those five cancer types currently account for about 50 percent of FoundationOne tests the company conducts, he added. Furthermore, Medicare and Medicare Advantage patients (who will also be covered by the NCD) account for about 40 percent of the company’s testing volume.
That should really help increase adoption which bodes well for investors in Foundation Medicine.
So far FMI has presented a rather tricky trade. In early 2015, shares popped nearly 100% on news that Roche was making a large investment in the company. We sold half our position at the time the news broke (about $50 a share), and congratulated ourselves at what geniuses we were when the share price fell to $14 a share in early 2016. Turns out, we should have backed up the truck when that happened.
If you bought shares of FMI at the beginning of this year, you’d be up over +252% on your investment at today’s price of $62.45 a share. Trying to make sense of these share price movements is futile, so it’s best to dollar-cost-average your way into a position if you’re interested in owning some shares. With a $2.2 billion market cap, FMI is still relatively small compared to the $207 billion market cap of Roche with whom they’ve climbed into bed. As with any investment, you need to pull the trigger based on your own convictions and have the fortitude to load up when everyone else is fearful.
Conclusion
Capitalism aside, maybe the biggest takeaway here is that if you or someone you love has cancer, see if you can get them a Foundation test. We were recently talking to some oncology experts about a unique lung cancer case we’re working on and they suggested “getting a broader genomic test like Foundation One which will measure the mutation burden and also screen for multiple rarer mutations that may be addressed via targeted treatments“. We received that bit of advice 3 days before FDA approval, so it seems more likely than ever that FMI’s test offerings are going to become the new norm.
Sign up to our newsletter to get more of our great research delivered straight to your inbox!
Nanalyze Weekly includes useful insights written by our team of underpaid MBAs, research on new disruptive technology stocks flying under the radar, and summaries of our recent research. Always 100% free.
















