A Prenatal Testing IPO from Ariosa Diagnostics
Table of contents
Table of contents

In a previous article, we highlighted Natera, a non-invasive prenatal testing (NIPT) company that has strong financial backing and a test that is said to consistently demonstrate high sensitivity and specificity for all major aneuploidies beyond Down syndrome. Although Sequenom (NASDAQ:SQNM) is the market leader in NIPT with more than 60% market share, there is plenty of growth to go around. A report published this year by Transparency Market Research, stated that the global NIPT market was just $220 million in 2012 but is expected to grow at a CAGR of 37.6% from 2013 to 2019 to finally reach $3.62 billion in 2019.
About Ariosa Diagnostics
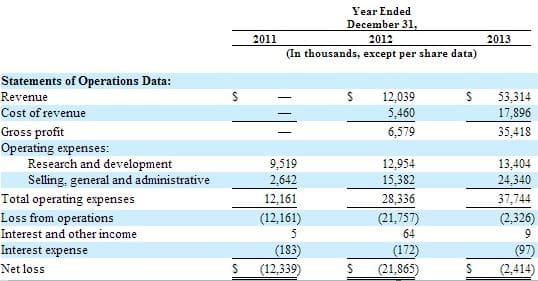
Founded in 2010, San Jose based Ariosa Diagnostics emerged from stealth mode in January 2012. In the same month, Ariosa secured more than $50 million in Series C funding from existing investors Venrock and Domain associates along with a new investor, Meritech Capital Partners. Venrock is the biggest shareholder of Ariosa at 39% with Domain following next at 24%. The executive chairman of Ariosa is John Stuelpnagel who co-founded Illumina (NASDAQ:ILMN). Mr. Stuelpnagel served 12 years at Illumina as the company’s first CEO and most recently as COO. Ariosa has cash on hand of $14 million and an accumulated deficit of around $40 million. Ariosa’s revenue growth is impressive, going from zero to $50 million in revenues in just 2 years with revenue increasing +343%, from 2012 to 2013:
The commercial launch of their only test offering, a prenatal test-marketed under the brand name Harmony, took place in March 2012. Since then, Ariosa has tested over 185,000 pregnant women, including over 45,000 women in the quarter ended December 31, 2013. This is a relatively small number compared to the potential market. Ariosa estimates that there are approximately 4 million births annually in the USA and approximately 130 million births annually outside the US. This means Ariosa had captured only 4.5% of the potential US market in their last quarter.
Harmony
Ariosa’s only test, Harmony, is a prenatal blood test that assesses the risk for chromosome conditions including Down syndrome (T21), Edwards Syndrome (T18), and Patau syndrome (T13). The test also includes an option to determine the gender of the fetus and also analyze sex chromosome (X,Y) conditions.

Harmony is offered in over 90 countries with approximately 25% of 2013 revenues generated in international markets. Ariosa has a non-exclusive agreement with LabCorp to provide logistics and sales and marketing support for Harmony in the United States and Canada. For 2012 and 2013, 98% and 73% of Ariosa’s revenue was from LabCorp. Ariosa does not have any issued patents covering Harmony but instead, exclusively licenses 6 patents that cover technologies for non-invasive prenatal testing. As of March 1, 2014, Ariosa’s patent estate included 37 patent applications pending in the United States, 31 of which are published. An article today by GenomeWeb discusses various legal encounters Ariosa has had in the past, in particular, one with competitor Sequenom. The federal court’s ruling last year invalidated US Patent No. 6,258,540 (Non-invasive prenatal diagnosis), which underlies Sequenom’s MaterniT21 Plus test which competes directly with Harmony. This was seen as a victory, a least temporarily, for Sequenom’s competitors.
Conclusion
Ariosa currently purchases the materials used in their laboratory process from Illumina (NASDAQ:ILMN). Given the executive chairman’s prior experience as an Illlumina co-founder, it seems likely he will be able to negotiate favorable pricing and maintain a strong relationship going forward with Illumina, a key supplier for Ariosa. With a rapidly growing market to address, strong proven leadership, and a fresh injection of capital soon to come, Ariosa is setting themselves up nicely to become a major player in NIPT. Ariosa intends to offer $69 million and trade under the symbol “AROS”.
Sign up to our newsletter to get more of our great research delivered straight to your inbox!
Nanalyze Weekly includes useful insights written by our team of underpaid MBAs, research on new disruptive technology stocks flying under the radar, and summaries of our recent research. Always 100% free.
















I think around 70,000 non-invasive prenatal tests are performed annually in Canada, maybe I am wrong. what do you think?
Thank you for the comment. Do you mean tests performed by Ariosa in Canada or just Canada tests administered overall? How did you reach that number either way?
I think you should check your math in the article with respect to Ariosa capturing 4.5% of the market in the last quarter. Ariosa processed 45,000 tests in the last quarter. All international tests were processed in the US and are included in that number. If you consider the 130 million annual birth total available market, then they captured about 0.14% of the market. If you were referring to the US market of 4 million births, you’d have to estimate what portion of these blood samples were from women in the US. They state that 25% of the revenues were from international. Typically international tests have a lower market price than the US and their distributors incur additional costs are are reduced from the ASP. I would peg a fair estimate of the US portion as 2/3 with international at 1/3. That would put the US capture rate around 3%. Hope that helps! Looking forward to see how the market reacts to the IPO.
Thank you for both your comments mchilberg. I was in fact referring to US market share and made that edit, Your estimates look much more accurate when considering those additional factors. This is an interesting space and Ariosa’s rapid growth in revenues shows that their is a great deal of market share to be claimed in the coming years
I don’t disagree. I’ve been watching this space with great interest for a couple of years now. Not only will NIPT likely grow dramatically in the next few years, but I believe we are underestimating the overall economic impact. In the context of overall reproductive health, a number of other tests from carrier screens to miscarriage analysis are also becoming increasingly interesting. Natera has their Natera One carrier screen and does a better job than the rest with their miscarriage and preimplantation testing. Illumina has a very good CF screen as does Sequenom who also screens for RhD, SMA and others. Ariosa doesn’t appear to supplement Harmony yet which is likely a mistake longer term. My view is that the pregnancy testing starts with carrier screening and will naturally flow to NIPT and if needed miscarriage testing. A traditional customer acquisition model would tell us that it’s quite likely the MFM or OB/GYN and patient would use one company for all these tests unless there is strong evidence to suggest any one was inferior.
Note that there is a lot of bad market data out on this segment I’m starting to build my own. Eventually I’d like to have country market size, pricing and market share data. If you run into good sources, I’d appreciate a heads up. Happy to reciprocate. .
Just published a free market research report:
http://seekingalpha.com/article/2153913-market-outlook-non-invasive-prenatal-screening-poised-for-significant-growth
Thank you for the link Mchilberg. Your report has a great deal of research and due diligence that investors in the NIPT story will no doubt find very useful.
Shams-I don’t believe this is accurate. Canada has an annual births of approx 8% of the US annual births. Your estimate would suggest that over 20% of all births are opting for NIPT. Note that Canadian insurance does not reimburse at present which is why MaterniT21 isn’t offered. Sequenom generally only launches in markets where reimbursement is probable. Using a logical estimation approach: US tests in 2013 from all 4 vendors totaled about 275,000-300,000. Canada annual births are approximately 8% of the US. So if both markets had the same market penetration, that would calculate to approximately 20,000. Given that the tests were all launched first in the US and that insurance providers are now covering the cost in the US, I would argue that Canada is likely less than half that or at most 10,000 tests last year. Hope that helps.
PS all the tests from Canada are being processed by labs in the US so they are likely already being counted in the numbers quoted by Ariosa in their S-1
I did a quick check. Tthe US birth rate quoted by Ariosa of 4m is accurate. Canada was about 375k. Note also that the Ariosa high risk quote appears understated. They appear to be using advanced maternal age only. That category represented 590 thousand in 2012 or 14.9% of births. Other risk indicators were ignored. Sequenom has estimated an additional 140 thousand annual births (3.5%) that would qualify as high risk under insurance carrier guidelines
Both risk categories are growing relative to annual births as women are waiting to give birth and molecular testing is getting more sensitive in providing indications of increased risk.
Sorry to see the terms were pulled for the Ariosa S-1. Will have to wait and see what their net step is. Sequenom had their earnings conference call tonight. Good progress on most fronts. Now they are arguing the market is clearly going to bifurcate into the full genome tests like MaterniT21 Plus/Verifi and Harmony for the targeted. References made to MaterniT21 Plus being a “virtual microarray” test.
Thank you for the heads up mchilberg. The below article talks a bit more about this. Apparently Illumina said it had filed a patent infringement lawsuit against Ariosa, which is both a customer and a competitor.
http://www.bizjournals.com/sanjose/news/2014/04/29/ariosa-diagnostics-removes-ipo-terms-faces-lawsuit.html?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+bizj_sanjose+(Silicon+Valley+%2F+San+Jose+Business+Journal)
FYI: Ariosa was acquired by Roche in December 2014:
http://www.xconomy.com/san-francisco/2014/12/02/roche-enters-noninvasive-prenatal-test-market-with-ariosa-purchase/