Buy Pre-IPO Shares in a Nano Drug Delivery Company
Table of contents
At the CB Insights fintech conference that wrapped up recently, an interesting fellow named Michael Novogratz made the remark that “Great bubbles occur around stories that are going to happen“, and we thought that would pretty appropriately describe the little hype bubble that took place around 2004 for nanotechnology. We had some forums at that time filled with people talking about “investing in nanotechnology” and before long it turned into a isht show of stock promoters, all of whom we exposed. We learned how to spot scams, and we learned that just because a technology is cool, it doesn’t mean that it’s “investable”.
What Happened to Nano Drug Delivery?
One theme we were all excited about back then was “nano drug delivery”, and there were a few stocks back then like Skye Pharma and Flammel that were working on nano drug delivery. While both of those companies have disappeared via mergers, today we have the opportunity to buy pre-IPO shares in a nano drug delivery company. Let’s see what this is all about keeping in mind that one nano drug delivery company that blew up recently, Bind Therapeutics. The company planning an IPO is called Kala Pharmaceticals.
A Kala Pharmaceticals IPO

Now, since these mucous membranes act as a barrier between the outside air and the human body, they serve to protect you in a similar way that your skin does. When you cut your skin and get an infection, it’s because there’s no barrier between the germs that surround us and the inside of your body. It makes sense then that penetrating the barrier of mucous membranes would be a challenge for drug delivery. This is where the nano drug delivery part comes into play.
In 2000, the John Hopkins Group set out to develop nano particles that could cross mucous layers and release drug compounds. They designed these nano particles after certain viruses that are known to be able to penetrate the mucous barrier. After sexually assaulting a fair number of mice, they were able to prove that their nano particle was suitable and then they gave Kala Pharmaceuticals an exclusive worldwide license to the technology which leads to the next part of the story.
With an exclusive license to a new nano drug delivery method, Kala Pharmaceuticals needed to figure out the most economically viable application of this technology so that they could make some money with it. They decided to solve a very simple problem which involved eye drops that are used after eye surgery.
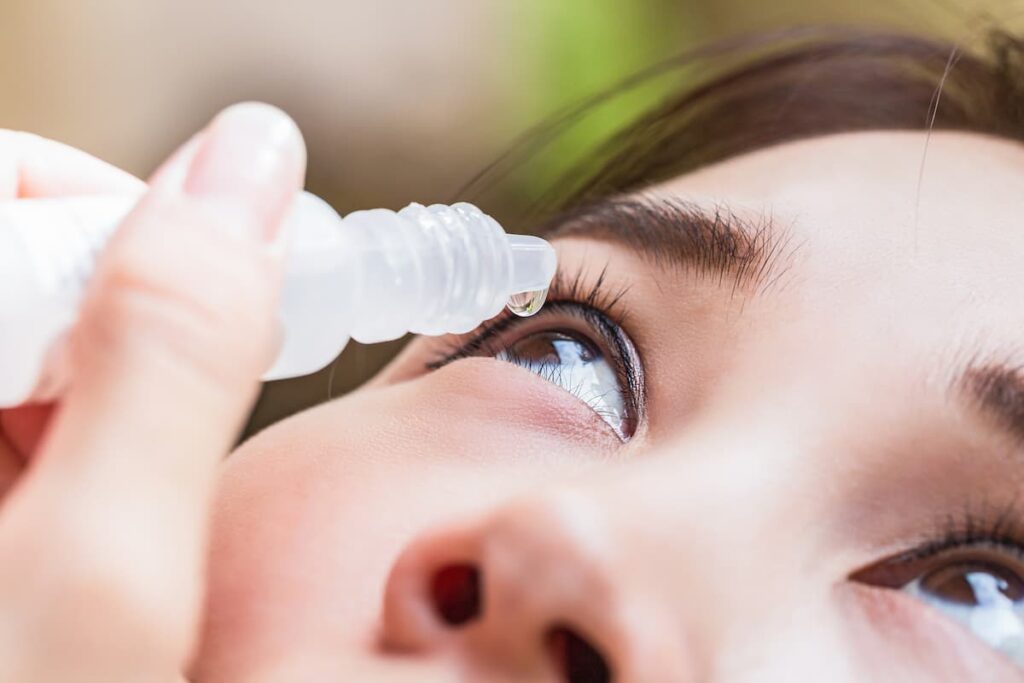
The most common eye surgery (also called ocular surgery) that takes place is known as cataract surgery. With upwards of 3 million surgeries taking place a year in the U.S., it’s one of the most common surgeries with 98% of patients experiencing improved vision as a result. That’s because they actually open up your eye and stick an artificial lens in there. After doing so, you can only imagine that your eye(s) are not going to be happy so you need to use these eye drops that contain steroids in order to help with the inflammation, eyedrops like these:
If you look at the active ingredient listed above, it’s something called “loteprednol etabonate” and that’s what Kala Pharmaceuticals decided to work with. They created “nanosuspensions” of that compound with their platform and found that you can use their eyedrops just twice a day, while the eyedrops you see above have to be administered 4X daily. Sounds like a pretty basic value proposition, but the first time you find yourself having to do 4X a day with eyedrops you’ll probably come to realize the appeal on offer from Kala.
In addition to creating their 2X daily post-surgery eyedrops, Kala is also addressing something called “dry eye disease” with the same compound at a lower dosage. Things are progressing so well for both of these applications that they’re already in Phase 3 testing as seen below:
With a solid pipeline of two Phase 3 candidates, we now need to get an idea of how much money they could stand to make.
The Total Addressable Market
This is where things start to get fun. We had one of our MBAs take a look at this and he started rambling on about TAM and Porters 5 Forces so we decided instead to use some common sense. TAM or “total addressable market” for a product tries to estimate just that, the total market you can sell into. In 2016 there were 7.7 million cataract surgeries, and let’s assume that each patient needed a bottle of eyedrops. We couldn’t find market share numbers for Altrex, but let’s assume that Kala captures a 25% market share with their product. The next key variable is price, and we were simply floored by how expensive these things are. Look:
So if Kala captures a 25% market share (1.9 million bottles of eyedrops), assuming a price point of $200 that’s around $385 million a year in revenues. Bear in mind that we’re only talking about one application here, eyedrops for post-surgery usage. The other application is something called “dry eye disease” which Kala estimates as affecting somewhere around 33 million people in the U.S.. The common treatments for dry eye disease are over-the-counter eyedrops called “artificial tears” and two prescription products – Restasis and Xiidra. In 2016, Restasis had sales of around $1.42 billion in the U.S. alone. As of last month, Kala had over 1,500 patients using their eyedrops in 2 separate Phase 3 trials. If those are successful, they look to file an NDA in the first half of 2018.
As with any pharmaceutical company, the risks are very high for investors. In this case, Phase 3 trials are promising and the total addressable market seems quite large with many potential applications down the road. The business model is easy enough to understand for a retail investor with no medical background so it’s hard for the company to pull the wool over your eyes if things aren’t working out. We’re going to probably pick up a small lot of pre-IPO shares in our Motif Investing account.
Conclusion
You can also buy pre-IPO shares for Kala Pharmaceuticals right now on the Motif Investing platform so get over there and open an account if you want to get some shares before the IPO. If you waited too long to do so, open an account anyways because Motif Investing has been very active lately in pre-IPO offerings.
Sign up to our newsletter to get more of our great research delivered straight to your inbox!
Nanalyze Weekly includes useful insights written by our team of underpaid MBAs, research on new disruptive technology stocks flying under the radar, and summaries of our recent research. Always 100% free.


















Alrex is an allergy medication. You invalidated your entire article my comparing an allergy drop to surgery drop. I count 6 versions of this me too product. Best of luck launching this product when Lotemax (original) was seldom used in cataracts. Soft steroid…